What Is The Coulter Principle And Why You Need To Obtain Complete Blood Counts By Flow Cytometry

When you get your blood drawn for your annual physical, or if you have been hospitalized, there are some common flow cytometry tests that your Medical Technician will run.
These tests will often analyze your blood lipids, vitamin levels, ion concentrations, and more often than not, your complete blood count (CBC).
The CBC is an automated hematology test (really several tests being run in the same instrument) that looks at the levels of all the cells in your blood, providing your physician with valuable information about your health.
The CBC is a powerful addition to many flow cytometry workflows.
Using just a small sample of blood, the CBC generates an extensive amount of information WITHOUT the need for centrifugation or multi-color staining experiments. Running a CBC is fast, easy, and inexpensive.
What Is The Coulter Principle?
The CBC generates accurate cell counts using the Coulter principle.
Named after its discoverer, Wallace Coulter, the Coulter principle states that particles passing through an orifice (along with an electrical current) will produce an increase in impedance, due to the displacement of electrolytes caused by the presence of the particle.
This change in impedance is proportional to the volume of the particle. The Coulter principle has been used for particle counting and sizing in a variety of fields, but one of the first and most impacted fields was hematology.
Labs that once took an hour to produce a blood count from a single patient using a microscope were now produced an order of magnitude faster and more consistently.
What Does A Complete Blood Count (CBC) Tell You?
A CBC, while inexpensive to run, gives you very valuable information. In addition, some CBC instruments only require a few microliters of blood to obtain an accurate reading.
For example, the Abbott Cell-Dyn 3500 requires about 200 µl of blood, but some newer instruments only require 15-20 µl of blood.
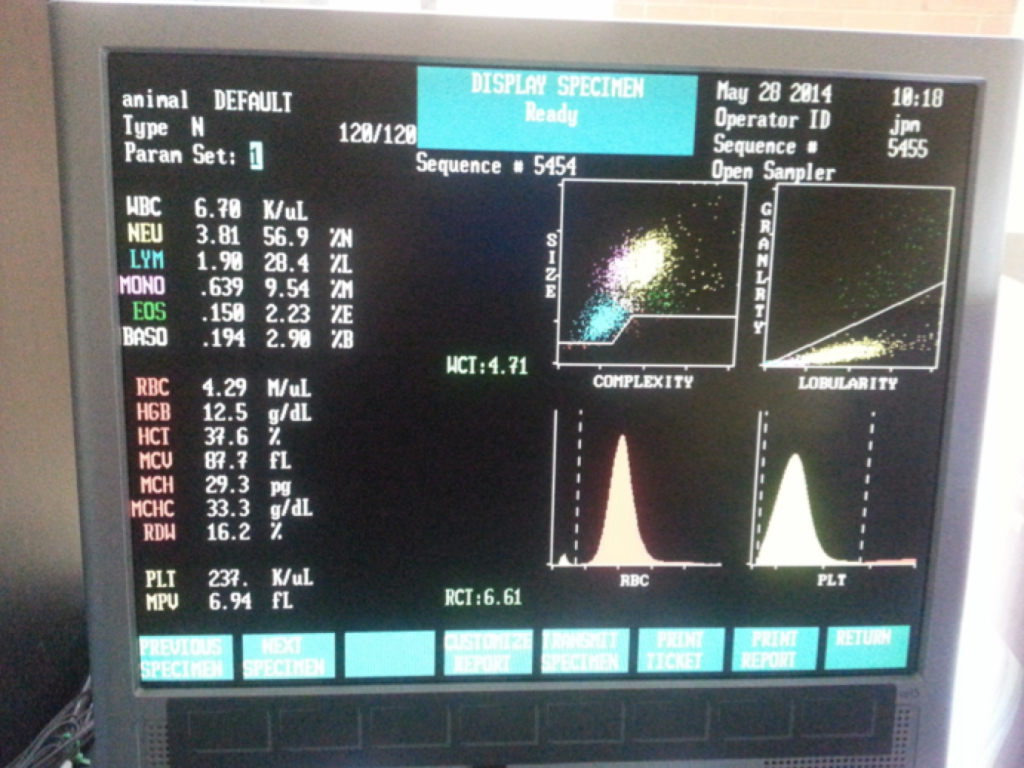
A typical CBC report will provide you with the following information…
1. Leukocyte count with differential—total white blood cell count and a 5 part differential (lymphocytes, monocytes, neutrophils, basophils, and eosinophils). Reported as total counts per microliter of blood and as a percentage of total leukocytes for the differential.
Depending on the instrument, this might only be a three part differential (lymphs, monos, and granulocytes). Instruments with optical counting in addition to impedance counting can give the 5 part differential.
2. Red blood cell count (also known as corpuscles).
3. Platelet count (also known as thrombocytes)—critical for blood clotting.
4. Platelet volume (measured in femtoliters).
5. Hemoglobin concentration (measured in grams per deciliter).
6. Hematocrit (also known as packed cell volume, expressed as a percentage measuring the total amount).
7. How much blood is cell volume versus plasma volume (expressed as a percentage).
8. Mean corpuscular volume (average volume of red blood cells).
9. Mean corpuscular hemoglobin and mean corpuscular hemoglobin concentration—these values are arrived at arithmetically using the red cell volume and hemoglobin results.
10. Red cell distribution width—variation in volume of RBCs.
How Can A CBC Help Me With My Flow Cytometry Experiments?
From your physician’s perspective, the CBC can reveal infections that might not yet be causing symptoms.
For example, elevated neutrophils could indicate a bacterial infection, elevated B and T lymphocyte function could indicate some viral infections or leukemia, elevated eosinophils could indicate a parasitic infection or an allergic reaction, and low red blood cells could indicate iron deficient anemia.
In the world of clinical research, a CBC should always be run on the human clinical research samples.
As a result, any obvious outliers can be removed from the study, reducing the spread of the data and reducing the risk of confounding your interpretation of the data. For investigators that wish to express their data in terms of count per volume of blood, running a CBC is one of the best ways to obtain accurate values.
You can lose somewhere between 10% and 20% of your cells with each centrifugation step, and that amount will vary depending on your buffer, cells, and whether or not you’re using glass or plastic tubes. Using counting beads is great for generating a total sample cell count, however, if your goal is to ascertain a whole blood count, it’s best to apply your gated fluorescent data statistics to the white blood cell count per microliter of total blood.
More Benefits Of Obtaining A CBC By Flow Cytometry
In addition to the enhanced accuracy and confidence a CBC provides, it’s also very fast.
Counting lysed blood via hemacytometer is tedious at best and performing it for more than a handful of samples is excessively time-consuming. Even image-based hand-held impedance counters are time-consuming, not to mention their decreased accuracy due to counting after lysis and centrifugation.) A Cell-Dyn or similar instrument, on the other hand, provides accurate results in 30 seconds.
Your mouse experiments can also benefit from CBCs. Mice with active infections might not be identified by animal tech welfare examinations but their increased neutrophil activity would certainly be identified by a flow cytometric CBC.
Another nice feature of many CBC instruments is the ability to set reference ranges for each reported value. In this way, low and high values are “flagged” on screen and in printed reports, allowing you to rapidly visualize and investigate any suspicious outliers.
CBCs also provide an additional layer of quality control in terms of how many cells you’re losing during your subsequent staining protocols. If you noticed that you’re losing more cells than you should be, you can investigate the problem by ensuring that everyone is following their standard operating procedures for lysis and centrifugation, testing a new lot of antibodies and other reagents, and so on.
Assuming you can spare the blood volume required, the CBC is a fast, easy, and inexpensive way to get additional data for your experiments. CBC experiments will allow you to quickly identify infections, outliers, and a myriad of cellular information. If you haven’t already invested in a CBC instrument, they are commonly sold on the refurbished market. Finally, in terms of cost per test, CBC reagents are also inexpensive, while quality control reagents are very reasonable as well.
To learn more about current best practices in flow cytometry, and to get access to all of our advanced materials including 20 training videos, presentations, workbooks, and private group membership, get on the Flow Cytometry Mastery Class wait list.

ABOUT TIM BUSHNELL, PHD
Tim Bushnell holds a PhD in Biology from the Rensselaer Polytechnic Institute. He is a co-founder of—and didactic mind behind—ExCyte, the world’s leading flow cytometry training company, which organization boasts a veritable library of in-the-lab resources on sequencing, microscopy, and related topics in the life sciences.
More Written by Tim Bushnell, PhD