3 Types Of Flow Cytometry Beads That Will Help Get Your Data Published

When starting a flow cytometry experiment, you should begin with the end in mind.
What data do you need to generate to support your other data? What data would really impress the reviewers in your study section and get your lab the grant you need? What data do you need to land your first tenure-track position?
Planning with your end goal in mind is important, but don’t forget the details along the way. Your cells are important, but ensuring your experiment is well set-up and your instrument properly maintained is just as important as elegant experimental design and good writing.
The 3 Best Flow Cytometry Beads
To make certain your instrument is set up correctly for your experiments, manufacturers have developed defined polystyrene beads.
These beads’ consistent nature helps you to assess how your instrument is behaving, helps you set up proper compensation matrices, and helps you generate volumetric counts of your cell populations. The best beads of the bunch in each of these 3 areas are highlighted below…
1. Quality control beads.
Manufacturers make certain that when your instrument is built and installed that the optical alignment is ideal. They do this with beads with multiple sizes and/or fluorescence levels, exactly like the ones you should be using. After installation and between preventative maintenance appointments, it is up to you (or your core staff) to track the performance of your instrument.
Alignment, sensitivity, and fluidic quality control beads should be run at least daily to ensure that with the same wattage on the laser and the same voltage applied to the detector you return the same median fluorescence (MFI).
Most new cytometers have an automated system to perform this activity, but even if yours does not, it is quite easy to generate your own Levy-Jennings plots.
There are several things to look out for when running your quality control beads. One thing to look out for is any change in the amount of voltage that needs to be applied to get the same MFI reading. Such a reading could indicate a loss of laser power if it occurs across all detectors for a given laser, or it could indicate a failing detector if the reading is isolated to a single PMT.
In addition, look out for changes in the coefficient of variation (CV) of your bead populations. Increased CV means less sensitivity and could point to misalignment of the laser, or issues with detection optics.
Our favorite quality control beads are BD’s CS&T beads and Spherotech’s multiple peak beads. The BD beads are really designed for their instruments using DiVa software, but they can certainly be used on other systems.
2. Compensation “capture” beads.
Compensation was a lot easier when you only had to work with FITC and PE. All you had to do was get some beads impregnated with those compounds, set your voltages, adjust your compensation matrix to correct for overlap, and voilà —you’re done.
Now, even beginners want to do 5+ colors. This makes antibody-binding compensation beads a necessity.
Beads with fluorochrome impregnated in the bead are still available, but compensation “capture” beads are ideal. The best of these beads bind antibodies of multiple isotypes from multiple species and give you a very bright positive signal from which to calculate your compensation matrix. These compensation beads also allow you to use the same fluorochromes you’re using in your experiment.
Using compensation capture beads is especially important when using tandem dyes as lot-to-lot variability and time-dependent changes in storage can change the spectral properties of these dyes.
“But my cells are free and work just fine!”
Sorry Charlie, you’re wrong.
One of the most important rules of good compensation is you want your compensation controls to be as bright, or brighter, than your actual experimental samples.
Beads will almost always be brighter (and therefore more accurate) in calculating a compensation matrix.
Another rule of compensation is that the colors have to be identical. If you’re using FITC in your experimental sample then you must use FITC in your compensation control. If you’re using tandem-dyes in your sample, you MUST use the same tandem dye in your controls, and so on. Using antibody capture beads ensures you’re using the same fluorochrome as is in your sample, ensuring compensation success.
One final note on compensation beads—compensation is the property of the fluorochrome, not the antigen.
Many novice flow cytometrists will make the mistake of staining the compensation beads with the same amount of antibody as is on their cells. This can lead to disaster, especially when the compensation control is off-scale on the instrument. If this happens, avoid the temptation to decrease the PMT voltage as this will just reduce the sensitivity on your cells (where it really matters).
The real solution is to reduce the amount of fluorochrome on the beads. We recommend you use 1/10 the amount of antibody on the beads as you do on the cells. Of course, this may vary depending on the antibody, and you must still make sure that the compensation signal is as bright as the signal on your cells.
Once again, if your compensation control is off-scale—DO NOT TURN THE VOLTAGE DOWN. Instead, re-stain the beads with less antigen.
Our favorite compensation beads are eBioscience’s Ultracomp beads. A lot of companies make capture beads, but these are the only ones I have seen that have such a wide range of species and isotype binding. Otherwise, be very careful about the species binding characteristics of what you buy.
3. Counting beads.
Counting beads are beads that come in multiple sizes, with or without fluorescence. The key is that these beads are shipped at a defined concentration. This allows you to set your stop gate to acquire a set number of events and use the events to calculate back to your cell concentration in the original sample.
Our favorite counting beads are Life Technologies Accucheck beads. These and other counting beads come in fluorescent and nonfluorescent forms but the fluorescent ones tend to be best. The fluorescence makes them very easy to gate for compared to gating on their scatter signal alone.
As seen in the example below, you could have a hard time gating on your count beads by scatter alone, as they are very close to your cells.

It can be hard enough to design your experiment with the proper controls, harvest your samples and develop your protocols. Make sure that you have an instrument set up that is stable, a good compensation matrix, and if you are at all interested in true counts of cells, the beads to make that accurate. If you are in a core environment, odds are very good that they are already running quality control daily on all instruments, and many people are not concerned with volumetric counts, but if you take nothing else from this article, make sure you are using capture beads to generate your compensation matrix.
Bonus—PMT Voltage Optimization Beads
Another one of our favorite beads are the Spherotech peak-2 bead (that is the second bead of the 8 peak bead-set—RCP-30-5A-2). These beads are used to optimize the PMT voltage settings on a flow cytometer based on this paper by Maecker and Trotter (2006).
To use these beads, simply run a voltage series on each PMT (typically 300-900 volts) and plot the CV of the data versus the voltage range. As a result, you’ll generate data similar to the data below.
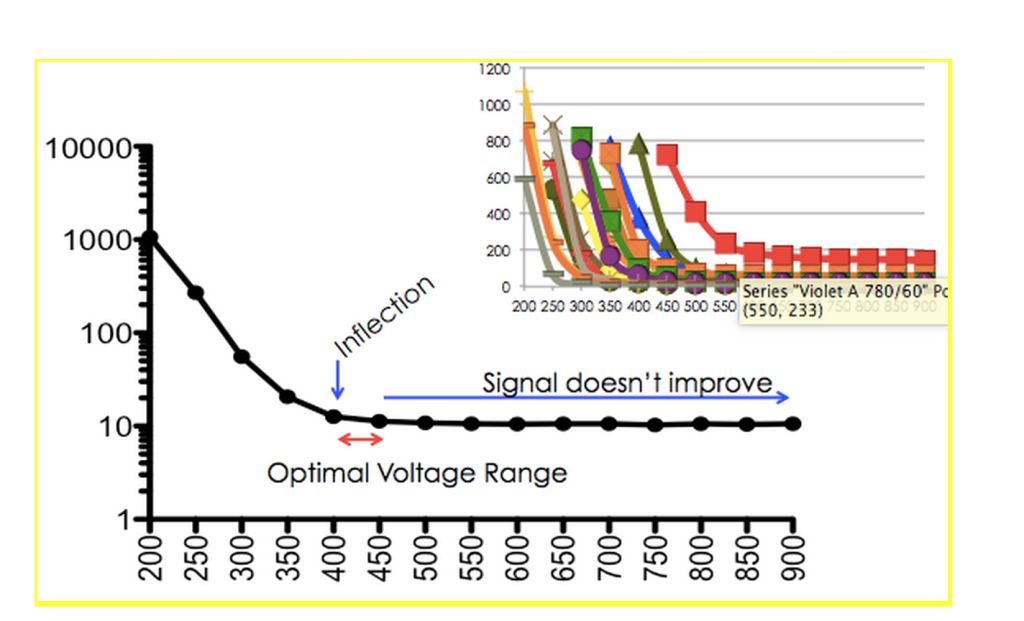
As voltage is increased, the CV of the beads (the measure of the spread of the data) decreases to an inflection point. At this point, the CV no longer increases, indicating that increasing voltages leads to increases in sensitivity of the detector.
The above flow cytometry beads will help ensure your experiment is well set-up and your instrument properly maintained. Alignment, sensitivity, and fluidic quality control beads will help you to ensure that with the same wattage on the laser and the same voltage applied to the detector returns the same MFI. The right compensation capture beads will bind antibodies of multiple isotypes from multiple species and give you a very bright positive signal from which you can calculate a correct compensation matrix. Finally, the use of counting beads allows you to easily calculate your cell concentration in your sample. Together, these flow cytometry beads will make your life easier and help you get your data published.
To learn more about the right flow cytometry reagents to use for your experiments and to get access to all of our advanced materials including 20 training videos, presentations, workbooks, and private group membership, get on the Flow Cytometry Mastery Class wait list.

ABOUT TIM BUSHNELL, PHD
Tim Bushnell holds a PhD in Biology from the Rensselaer Polytechnic Institute. He is a co-founder of—and didactic mind behind—ExCyte, the world’s leading flow cytometry training company, which organization boasts a veritable library of in-the-lab resources on sequencing, microscopy, and related topics in the life sciences.
More Written by Tim Bushnell, PhD












