Measuring Receptor Occupancy With Flow Cytometry

The field of medical therapeutics is moving into the area of precision medicine. In a global sense, precision medicine requires the doctor to assess a patient’s unique disease state — the susceptibilities and resistances of the disease targets to the arsenal of medicines at the physician’s disposal.
This is leading the push towards devising more nuanced tools, and an understanding of what specific patient characteristics dictate which tools to use.
For precision medicine to work, we must be able to identify biomarkers that are expressed on diseased cells, but absent on the normal cell.
An example of this type of biomarker is overexpression of Her2 on a subset of breast cancers. The drug Herceptin targets the Her2 overexpressed on these cells. Studies suggest that the binding of Herceptin induces an immune response, as well as causing a G1 arrest, reducing cell proliferation.
The success of drugs like Herceptin and Rituximab is one of the reasons there are hundreds of drugs of this class in development.
The ability to perform quantitative assays in a phenotypically defined cell population, and the ease of moving to high-throughput assays, means that flow cytometry is the assay of choice for assessing the performance of these drug candidates, especially as they enter into pre-clinical trials.
Assessing the appropriate dosing for pre-clinical trials is an especially important step. Too low a dose and the data is not compelling, too high and you run the risk of adverse reactions in your subjects.
While these decisions are based on data from animal models, there is not always good correlation between the two.
Take, for example, this report from a phase 1 clinical trial of an anti-CD28 antibody, TGN1412. Healthy subjects were infused with the drug, at a concentration that animal studies suggested would be only 10% receptor occupancy, which turned out to be over 90% in humans, leading to the subjects ending up in the ICU because of systemic inflammatory response syndrome.
Measuring Receptor Occupancy (RO) by flow cytometry seems a logical step, and has been receiving a great deal of attention in the clinical cytometry world. So much so, that Cytometry B devoted a special issue to this topic.
There are 3 common ways to measure RO, either directly or indirectly. Each provides a different type of data, and the experimental needs will dictate what assay to perform. An additional consideration for method choice is the ability of directly conjugated reagents.
1. Measuring total receptor expression
In this assay, a labeled, non-competing antibody is used to label the cells. This antibody should bind to the same antigen as the target antibody, but not to the same epitope. Using this assay, it is possible to obtain a measure of the total expression on the surface of the cell.
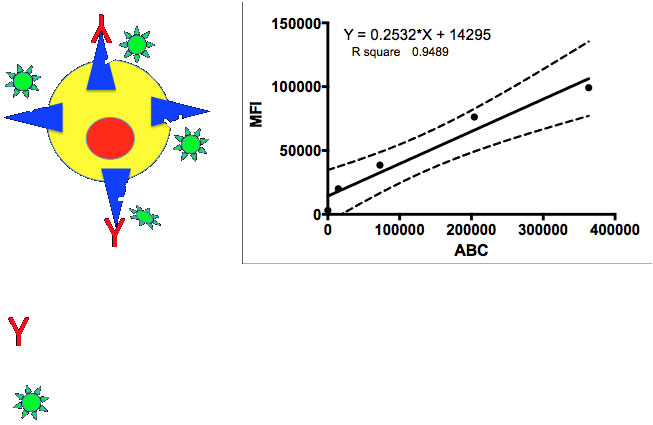
Figure 1: Theoretical measurement of total receptor expression. The presence of the target antibody is irrelevant, the non-competing Ab measures the total expression in terms of antibody binding capacity (ABC). A standard curve is generated using beads with known quantities of ABC and the MFI related to the ABC on the target cells in an experiment.
2. Measuring free receptor expression
In this experiment, the target antibody is labeled. Cells are first labeled with the unlabeled target antibody. This is followed up with the labeled target antibody, which will only bind to free sites. Again, using a standard curve, it is possible to measure the number of free receptors.

Figure 2: Theoretical measurement of free receptor expression. After incubating the cells with the target antibody, the cells are incubated with a fluorescently labeled target antibody. A standard curve is generated using beads with known quantities of ABC, and the MFI related to the ABC on the target cells in an experiment.
3. Measuring receptor occupancy
If the first two assays are combined, it becomes possible to monitor the occupancy of a receptor over time. This is especially useful, as many of the monoclonal antibodies in use have long biological half-lives, and understanding their kinetics is critical for making therapeutic decisions.

Figure 3: Measuring receptor occupancy by flow cytometry. Cells are first incubated with the target antibody. This is followed by incubation with fluorescently labeled target antibody and the non-competing antibody. After calculations of the ABC, the average receptor occupancy can be calculated.
Of course, if this experiment is performed over several days, curves and kinetics of binding can be readily calculated. There are several different ways this data can be plotted, depending on the question being asked.

Figure 4: Changes in receptor occupancy over time, as measured using the assay in Figure 3.
As with every clinical assay, there are a host of additional concerns that need to be addressed in the experimental setup: assay validation, optimization, and more. A comprehensive review of these steps can be found here, and is recommended reading for anyone seeking to integrate receptor occupancy assays into their research.
In conclusion, measuring the receptor occupancy of a given target showcases the power of flow cytometry. With the right reagents, best practices, and attention to detail, this assay can become a mainstay in your research toolkit. It extends quantitative flow cytometry to the next level, to determine a complete biological picture of how efficiently a given target is being bound. This also serves as the basis for even more fine-analysis when combined with assessment of downstream targets that the engagement of the receptor by the target antibody may affect. Phosphorylation, cell cycle arrest, and protein expression are all within reach, resulting in an even more complete picture of the process that will ultimately give the medical community a fuller understanding of how these potential therapeutics work, and when to use them. This is truly personalized medicine at its fullest potential.
For further reading:
- Targeted Cancer Therapies at the NIH
- American Association of Pharmaceutical Scientists blog 1 June 2016
- Green et al (2016) Recommendations for the development and validation of flow cytometry-based receptor occupancy assays. Cytometry B 90:141-149
To learn more about Measuring Receptor Occupancy With Flow Cytometry, and to get access to all of our advanced materials including 20 training videos, presentations, workbooks, and private group membership, get on the Flow Cytometry Mastery Class wait list.

ABOUT TIM BUSHNELL, PHD
Tim Bushnell holds a PhD in Biology from the Rensselaer Polytechnic Institute. He is a co-founder of—and didactic mind behind—ExCyte, the world’s leading flow cytometry training company, which organization boasts a veritable library of in-the-lab resources on sequencing, microscopy, and related topics in the life sciences.
More Written by Tim Bushnell, PhD












