4 Considerations For Assessing Protein Phosphorylation Using Flow Cytometry

Signaling pathways are of great interest in many areas of research because dysregulation of these pathways can lead to diseases such as cancer and lupus. This also means these pathways contain potential therapeutic targets to help treat these diseases.
The power of flow cytometry is the ability to analyze millions of events in a short period of time. This allows for the analysis of dynamic cellular processes in a phenotypically defined manner.
The process of cellular signaling relies on changes in the phosphorylation state of proteins to either up or down regulate downstream processes (Figure 1).

Figure 1: The PI3Kinase signaling pathway has been implicated in cancer growth. From Vivance and Sawyer (2002) Nature Reviews Cancer 2:489-501
Initially, phosphorylation state analysis was performed by western blot — a bulk analysis method where subtleties of expression can be lost. To transition phosphorylation analysis to a flow cytometry assay, several factors needed to be optimized.
First, a source of high-quality antibodies to a very specific target needed to be produced. Second, the best way to stain the cells for specific, efficient labeling of targets while preserving the integrity of surface staining needed to be determined.
Proteins can be phosphorylated at 3 residues: Serine, Threonine, or Tyrosine. Phosphoprotein antibodies must be able to resolve a single change at the appropriate site in the presence of non-phosphorylated targets. When screening hybridomas, manufacturers must screen for both positive binding and absence of binding to the non-phosphorylated target.
The second concern was testing which protocols would be best for fixation and permeabilization of the cells to preserve the surface staining, the epitopes, and cell shape, while ensuring the antibodies were able to access the intracellular space.
If you are considering going after phosphorylation state, here are 4 important considerations that you should keep in mind when performing your experiments.
1. Choice of Instrument — while this may sound odd, there are 3 different classes of instrument on the market that can be used for cytometry-based assays. The traditional fluorescent flow cytometers, the spectral analyzers, and the mass cytometer. Each have their strengths when performing this assay.
- Traditional Fluorescent Flow — most readily accessible to the majority of users. There is a limited choice of fluorochromes for phosphoflow, which is impacted by the size of the fluorochrome and the autofluorescence of the cells. This limits the number of targets that can be measured in one assay.
- Spectral Analyzers — still relatively new to the marketplace. These systems should help improve resolution, as they are better able to handle autofluorescence of the cells. There are still limits because of fluorochrome size, but spectral analyzers can allow resolution of closely overlapping fluorochromes. As shown on this figure generated on the new Aurora from Cytek, they are able to resolve AF488 and AF532 in the presence of PE (Figure 2).

Figure 2: Emission resolution possible with spectral analyzers. Information from Cytek.
- Mass Cytometry — it is hard to beat the power and multiplexing capabilities of the CyTOF mass cytometer. Over 30 parameters are possible, and the use of isotope labeled antibodies alleviates size constraints. Likewise, there is no autofluorescence from the cells to reduce sensitivity. The high cost of adopting a mass cytometry panel, the limited availability of reagents, and potential data analysis complexity all reduce the attractiveness of this technology, but if you are seeking to screen a large number of targets, the mass cytometer is hard to beat.
2. Choice of Fluorochrome panel design for phosphoflow assay offers some additional challenges. First, is the consideration of the size of the fluorochrome.
After making holes in the membranes, it is important that the contents do not leak out, so the holes can’t be too large. We are trying to get antibodies into the cells, so imagine adding a PE molecule that is larger than a typical antibody and trying to get that into the cell.
That is why cyclic ring compounds like Fluorescein, Texas Red, and the Alexa type dyes are popular. These are relatively small (usually under 1000 g/mol), so don’t impact the size of the antibody as much as the larger fluorochromes do (Figure 3).
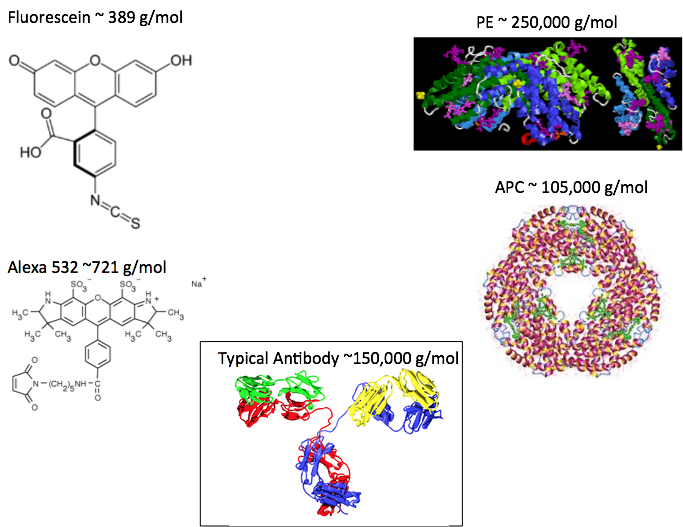
Figure 3: Size of some common fluorochromes including Fluorescein, Alexa 532 (carboxylic acid, succinimidyl ester shown), Phycoerythrin, allophycocyanin, and a typical antibody.
Once the fluorochrome choice is made, surface staining must be considered. This will be impacted in 2 ways.
First, the choice of phospho-antibody conjugate will remove some fluorochromes from consideration. Second, optimal fixation conditions for the phospho-antibody may negatively impact the surface stain fluor. Fortunately, there is a guide published by Cytobank to help make this decision.
3. Choice of Protocol — fixation and permeabilization protocols are critically important for success of these experiments. The same link from Cytobank can help with protocol choice. The choice of fixatives and the order that the fixatives are used is critically important to get right and optimize (Figure 4).
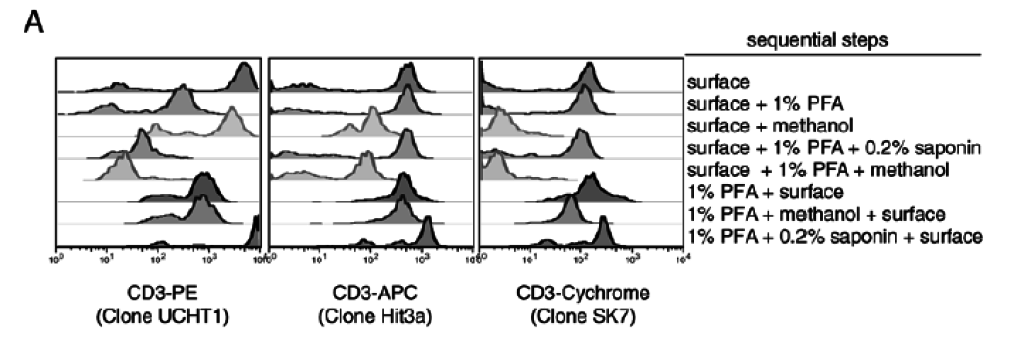
Figure 4: Effects of different fixation protocols on the surface staining of different clones and fluorochromes. From Perez et al., (2005) Curr Protoc Cytom. Unit 6.20.
4. Choice of Analysis — when done properly, this assay can generate a great deal of information quickly, and it is critical to find ways to present it both graphically and statistically. This can be done using heat-maps or other tools.

Figure 5: Heatmap analysis and clustering of 6 different phospho-Ab, either at basal or one of 5 different stimulation conditions. From Irish et al., (2004) Cell 118:217-228.
If you are interested, another way to look at this is a fold change. Jonathan Irish recommends using a transformed ratio, and in the simplest form it is:
log10(MFIstim) – log10 (MFIunstim)
Other data transformations exist, and these are discussed here.
Of course, the standard considerations for any flow cytometry experiment exist, such as appropriate samples, proper simulation, the necessary controls, and the protocols for setting up and running the instrument. Phosphoflow adds a level of complexity to this process, but it is not insurmountable.
If you are seriously considering adopting this assay into your research, it may be good to read and take the U937 challenge. This challenge is a perfect training tool to see how well you can perform and analyze phosphoflow data.
If you want more training, the Irish lab at Vanderbilt has put their training material on the web for everyone to use. You can also find some videos at the Nolan lab at Stanford.
For those working in the signaling field, having the ability to take a sample and phenotypically identify it, while knowing what is happening inside the cell to the target molecules of choice, opens up a host of new opportunities. These assays are amenable to high throughput setup, meaning that biologically relevant outcomes in pre-clinical drug discovery can be measured directly. All told, with a little forethought, some careful planning, and validation, phosphoflow assays are within your reach.
To learn more about the 4 Considerations For Assessing Protein Phosphorylation Using Flow Cytometry, and to get access to all of our advanced materials including 20 training videos, presentations, workbooks, and private group membership, get on the Flow Cytometry Mastery Class wait list.

ABOUT TIM BUSHNELL, PHD
Tim Bushnell holds a PhD in Biology from the Rensselaer Polytechnic Institute. He is a co-founder of—and didactic mind behind—ExCyte, the world’s leading flow cytometry training company, which organization boasts a veritable library of in-the-lab resources on sequencing, microscopy, and related topics in the life sciences.
More Written by Tim Bushnell, PhD












